|
Uranium Life-Cycle
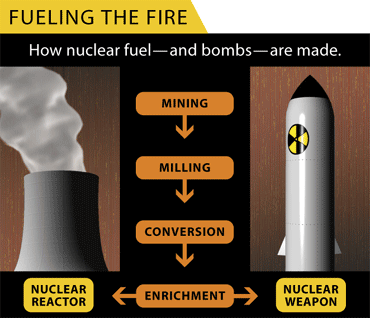
MINING:
Uranium, the essential raw material of both civilian and military nuclear programs, occurs in nature as a hard, radioactive, metallic element. It consists of two isotopes, mostly U-238 (99.3 percent), and U-235 (0.7 percent). Uranium mining is among the most carbon-dioxide-intensive operations in the world.
MILLING:
Uranium ore in the United States. contains between 0.05 to 0.3 percent uranium oxide (U3O8). The ore is crushed and soaked with sulfuric acid to leach out the U308. When dry it is powdery and yellowish and commonly known as "yellowcake."
CONVERSION:
Because uranium needs to be in gaseous form before it can be enriched, the yellowcake is heated to about 147 degrees Fahrenheit, at which point it is converted into uranium hexafluoride gas (UF6). This compound is corrosive to most metals (but only mildly to aluminum), highly toxic, and violently reactive with water.
ENRICHMENT:
The UF6 gas is pumped into a centrifuge that spins on an axle at the speed of sound, creating artificial gravity. The slightly heavier U-238 moves to the outside of the tube while the lighter and highly fissionable U-235 concentrates toward the center of the tube, where it is collected. This enriched U-235 continues to be fed into a chain of other centrifuges, a process known as a cascade. The enrichment process removes about 85 percent of the U-238 by separating the UF6 into two streams. Both streams are still primarily U-238 but one stream has a lower concentration of U-235 than the other. This stream is essentially U-238 with all the U-235 removed and is referred to as depleted uranium, tails, or waste; it is stored in steel cylinders, each containing up to 12.7 tons of the radioactive material.
NUCLEAR REACTOR:
The higher-concentration stream continues on its enrichment route. For nuclear reactor fuel, the uranium is enriched to contain 3 to 5 percent U-235. This enriched uranium is then converted into uranium dioxide (UO2) powder, pressed into small pellets-each roughly the size of a coin and about an inch long-and then placed into long fuel rods for use in commercial nuclear reactors. The rods form the core of the nuclear-power reactor. After the rods are used for 12-18 months, their metallic outer casing is stripped away and they are dissolved in hot nitric acid. The process produces uranium (96 percent), which is reprocessed for reuse in reactors; highly radioactive waste (3 percent); and plutonium (1 percent). Experts believe terrorists who gain access to plutonium could create a crude bomb using skills no greater than those used by the Japanese cult that manufactured nerve gas and attacked the Tokyo metro in 1995. A nuclear explosion using plutonium would be 20 times more powerful than the largest terrorist bomb attack to date.
NUCLEAR WEAPONS:
The same enrichment process used to make nuclear-reactor fuel continues. Enriched U-235 moves down a series of about 1,500 centrifuges. At 20 percent purity, the uranium is considered "highly enriched uranium" (HEU). It takes about a year to enrich U-235 to weapons grade, or 90 percent pure, uranium-which can then form the core of a nuclear bomb. The higher the enrichment level, the less HEU is needed to make a bomb. The simpler "gun-type" weapon uses 90-110 pounds of HEU and could likely be built by some terrorist groups. The "implosion-type" weapon only needs 33 pounds of HEU but is more technically difficult to make.
Up to Top
|